What is Aluminum / Anodize? |
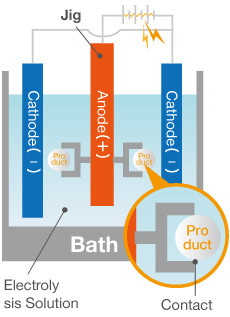
Anodizing is a surface treatment process in which aluminum (anode) is electrolyzed to artificially generate an oxide film (rust).
Aluminum easily reacts with oxygen, creating an extremely thin oxide film when in contact with air. This naturally produced film forms a protective layer that prevents rust, resulting in aluminum’s characteristically good corrosion resistance. However, this film is extremely thin and can corrode due to environmentally-induced chemical reactions, necessitating a protective surface treatment. That is where anodizing comes in.
Basic Structure |
|
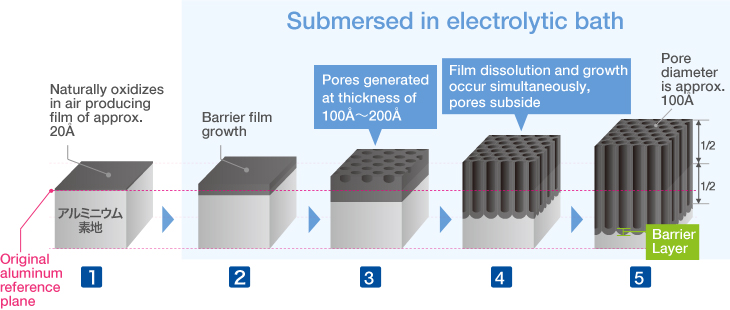
Anodizing Treatment Process
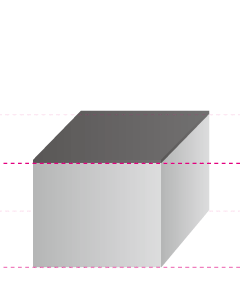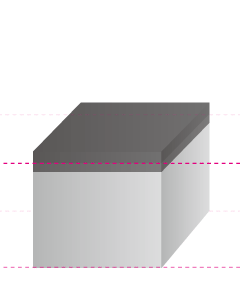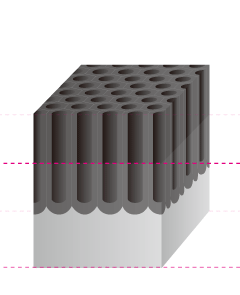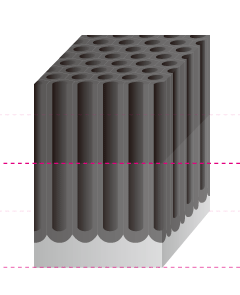
The Basic Structure of Anodizing (anodizing) is a Bunch of Pencils!? Electrical induction of aluminum melts the surface, simultaneously generating osmotic film and oxide film to form 3-dimensional cells over time. It may be helpful to think of the basic structure as a bunch of hexagonal pencils sprouting from the surface of the aluminum. |
 |
|
|
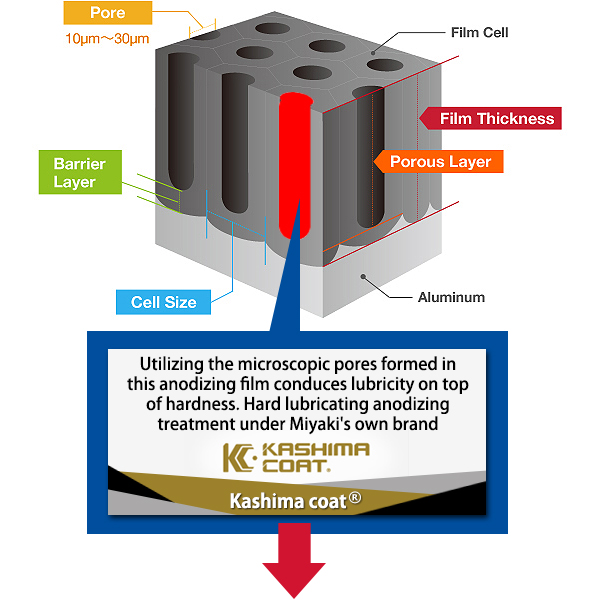
Pore Size
The term pore refers to the holes in the oxide film that are 100Å-300Å(Angstrom) in diameter. There are actually an amazing 5-70 billion of them per cubic centimeter. The entire population of the world could easily fit in this small space if shrunk down to the atomic level.
About Kashima Coat |
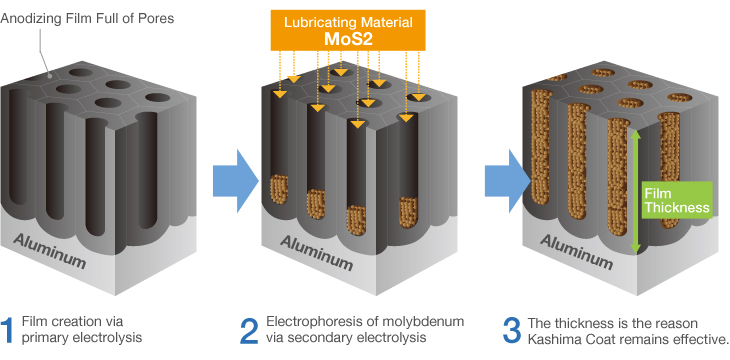
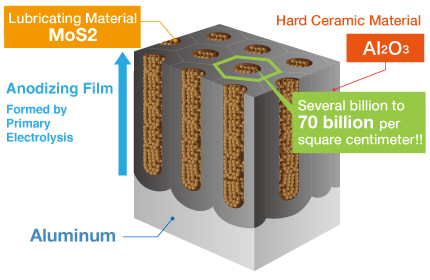
Kashima Coat is lubricated anodizing developed by Miyaki Corporation by adding a lubricating function to hard anodizing with the goal of enhancing wear resistance.
The repetition of “hard + lubricant” shown in the diagram produces up to 70 billion molecules per square centimeter that significantly improves the wear resistance of Kashima Coat in comparison to hard anodizing.

|
Kashima Coat is lubricated anodizing developed by Miyaki Corporation by adding a lubricating function to hard anodizing with the goal of enhancing wear resistance. The repetition of “hard + lubricant” shown in the diagram produces up to 70 billion molecules per square centimeter that significantly improves the wear resistance of Kashima Coat in comparison to hard anodizing. Kashima Coat Treatment Process Kashima Coat is created by filling the countless regularly aligned pores of the anodizing(anodic oxide) film generated via primary electrolysis with the lubricating material molybdenum disulfide. |
 |